Abstract

Background: Real-world data about the effectiveness of SARS-CoV-2 vaccination and implementation of booster campaigns in an immunocompromised population such as patients diagnosed with hematologic malignancies (HM) are scarce.
Our aim was to compare morbidity and mortality of COVID-19 according to SARS-CoV-2 vaccination status and immunological response to vaccination in a cohort of patients diagnosed with HM, considering a scenario affected by the succession of diverse virus variants and the advent of COVID-19 specific therapies.
Methods: We retrospectively collected clinical data from the patients with a current or prior history of HM at Vall d´Hebron Universitary Hospital who suffered a laboratory-confirmed SARS-CoV-2 infection between March 2020 and May 2022.
The main endpoints included severity of COVID-19 (WHO classification), rate of hospitalization, ICU admission, need for supplemental oxygen or COVID-19 specific therapy (steroids, antivirals, IL-6 inhibitors, hydroxychloroquine, anti-SARS-CoV-2 monoclonal antibodies). We also genotyped viral variants and assessed humoral immunogenicity induced by the vaccine (anti-spike[S] IgG >260 BAU/mL measured by LIAISON® Diasorin) or the infection (anti-nucleocapsid[N] IgM/IgG/IgA positivization by Elecsys® Roche).
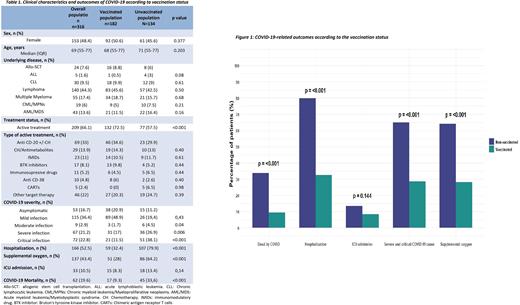
Results: A total of 326 patients developed COVID-19, 182 vaccinated cases between 03/21 and 05/22, and 134 unvaccinated cases between 03/20 and 02/22. Both groups were similar in age (median 68 and 71 years) and gender (51% and 46% women, respectively). In both cohorts the main diagnosis was lymphoma (46% and 43%), the majority of patients were under active treatment (73% and 58%) and the most frequent active therapy was anti-CD20 monoclonal antibodies+/- chemotherapy (35% and 30%) (Table 1).
Among the 113 vaccinated sequenced cases, 80% were omicron (predominantly BA.1 [36%] and BA.1.1 [28%]), 19% delta (B.1.617.2 [6%] and AY lineage [15%]) and 1% alfa variant. Genotype analysis of 87 unvaccinated cases showed predominance of B.1 and B.1.177 lineages in 53% and 35% of the cases, respectively.
The vaccines most frequently administered were mRNA-1273 (70%) and BNT162b2 (25%). A 5% of patients were partially vaccinated (one dose), 26% fully vaccinated (2 doses), whereas 67% received a third boost dose and 2% a fourth dose prior infection.
Considering patients with available basal serology prior infection, only 20% (1/5) of partially vaccinated, 39% (13/33) of fully vaccinated patients, and 47% (42/90) of boosted patients had developed positive anti-S IgG antibodies. In 38% (34/90) of patients with basal negative anti-N antibodies a seroconversion was observed after the infection.
In the unvaccinated cohort the most frequent COVID-19 specific therapies administered were hydroxychloroquine (46%), steroids (28%), lopinavir/ritonavir (20%) and tocilizumab (19%); while in the vaccinated cohort were remdesivir (38%), steroids (28%), tocilizumab (14%) and anti-SARS-CoV-2 monoclonal antibodies (7%).
In comparison with the unvaccinated cohort of patients, vaccinated patients had less severe (17% vs. 27%; p=0.006) and critical (12% vs 38%; p=<0.001) COVID-19, lower rates of hospitalization (32% vs. 80%; p=<0.001) and fewer patients needed supplemental oxygen (28% vs. 64%; p=<0.001). Admission rates to ICU were similar between both cohorts. Mortality rate was lower in the vaccinated patients in comparison with the unvaccinated cohort (HR: 0.48 (95%CI 0.31-0.74); p=<0.001) (Figure 1).
Of note, only 15% (6/39) of patients who suffered a severe/critical infection had developed humoral vaccine immunogenicity. In contrast, 56% (50/89) of mild/moderate cases had created vaccine-induced seroconversion. None of the 17 vaccinated patients who died had developed anti-S antibodies.
Finally, in 23% (30/131) of the vaccinated cohort the follow-up RT-PCR remained persistently positive >21 days.
Conclusion: In this real-world analysis, and despite the heterogeneity of infection by different viral variants and treatment availability for COVID-19 during the evolution of the pandemic, vaccination has resulted widely effective at reducing need of hospitalization, severity and mortality of the SARS-CoV-2 infection in patients with HM. Patients who develop a suboptimal humoral immunogenicity after vaccination are at higher risk to develop a severe/critical infection and would benefit from an early intervention.
Disclosures
Cabirta:Jazz: Honoraria; Janssen: Honoraria; Astra-Zeneca: Honoraria. Abrisqueta:Janssen: Consultancy, Honoraria, Speakers Bureau; AstraZeneca: Consultancy, Honoraria, Speakers Bureau; BMS: Consultancy, Honoraria, Speakers Bureau; AbbVie: Consultancy, Honoraria, Speakers Bureau; Roche: Consultancy, Honoraria; Sandoz: Honoraria; Incyte: Consultancy. Bosch:Karyospharm: Honoraria; Celgene/BMS: Consultancy, Honoraria; Roche: Consultancy, Honoraria; Takeda: Consultancy, Honoraria; Astra Zeneca: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; Mundipharma: Consultancy, Honoraria; Gilead: Consultancy, Honoraria; Abbvie: Consultancy, Honoraria; Lilly: Consultancy, Honoraria; Beigene: Consultancy, Honoraria.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal